Se Cation Or Anion
The key difference between anion and cation is that anions are the negatively charged ions formed from neutral atoms whereas cations are positively charged ions formed from neutral atoms. Commonly, anions and cations are called ions. The atoms of various elements are not stable (except the noble gases) under normal conditions. Each question's answer is either 'cations' or 'anions'. Terms in this set (12) Give up electrons to reach octet. Negatively-charged ions. Form ions that are larger than their original atoms. If valence is high or close to 8, an atom will form. Complete List of Cation and Anions. Selenate SeO4 2-, Selenide Se 2-, Selenite SeO3 2-, Sulfate SO4 2-, Sulfide S 2-, Sulfite SO3 2-, Tartrate C4H4O6 2-, Tellurate TeO4 2-, Telluride Te 2-, Tellurite TeO3 2-, Thiosulfate S2O3 2-, Titanate TiO3 2-, Tungstate WO4 2. Dietary cation–anion balance is important in animals to maintain systemic acid–base balance and osmotic pressure in order both to protect the integrity of cells and membranes and to optimize biochemical and physiological processes. Dietary cation-anion balance and cation source effects on production and acid-base status of heat-stressed cows. Journal of Dairy Science 75, 2776 – 2786. West, JW, Mullinix, BG and Sandifer, TG 1991.
Main Difference – Cation vs Anion
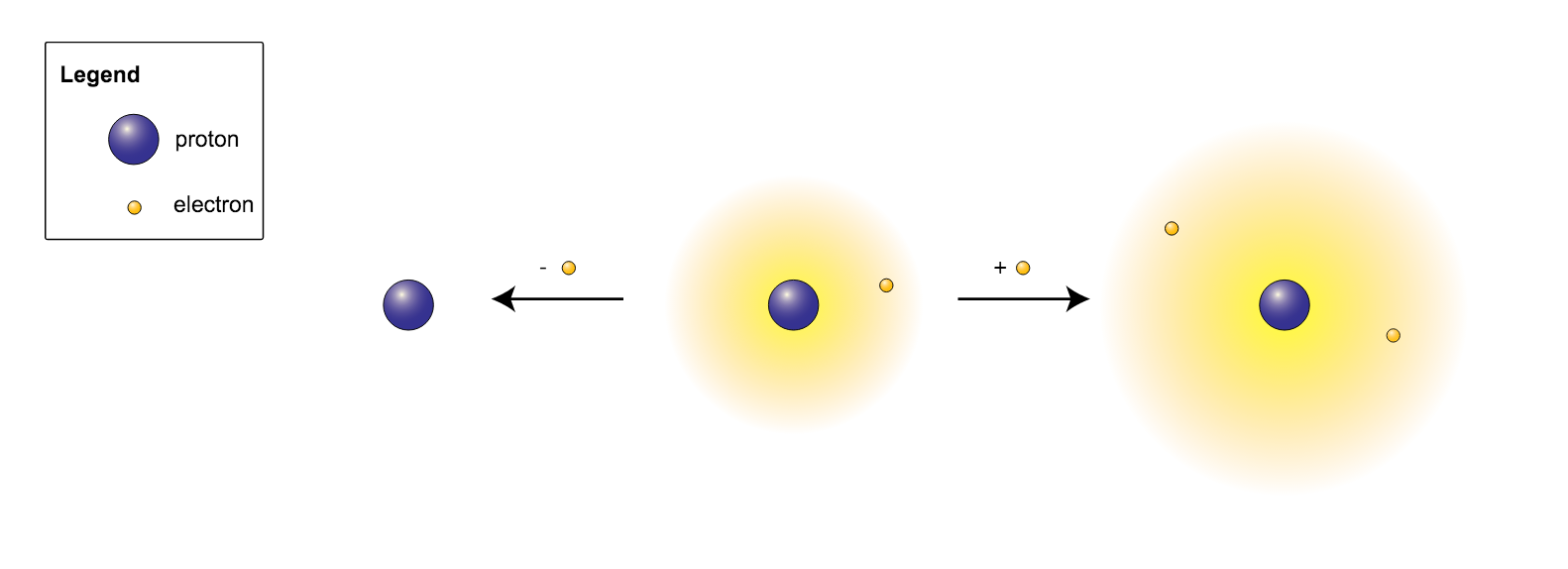
Cation and Anion are opposite terms in chemistry and stand for the two main types of ions formed. An ion is a state of matter upon the loss or gain of electron(s) compared to its actual state. When elements remain in their original form, they are known as ‘atoms’. However, most of the elements do not remain in their atomic state in nature; they tend to either acquire or giveaway electrons in order to attain stability like the noble gases. As a pre-step for the formation of compounds, these atoms transform them into positions or states of matter called ‘cations’ or ‘anions’. A cation is formed when an atom gives away electrons; an anion is formed when an atom acquires electrons. This is the main difference between cation and anion.
What is a Cation
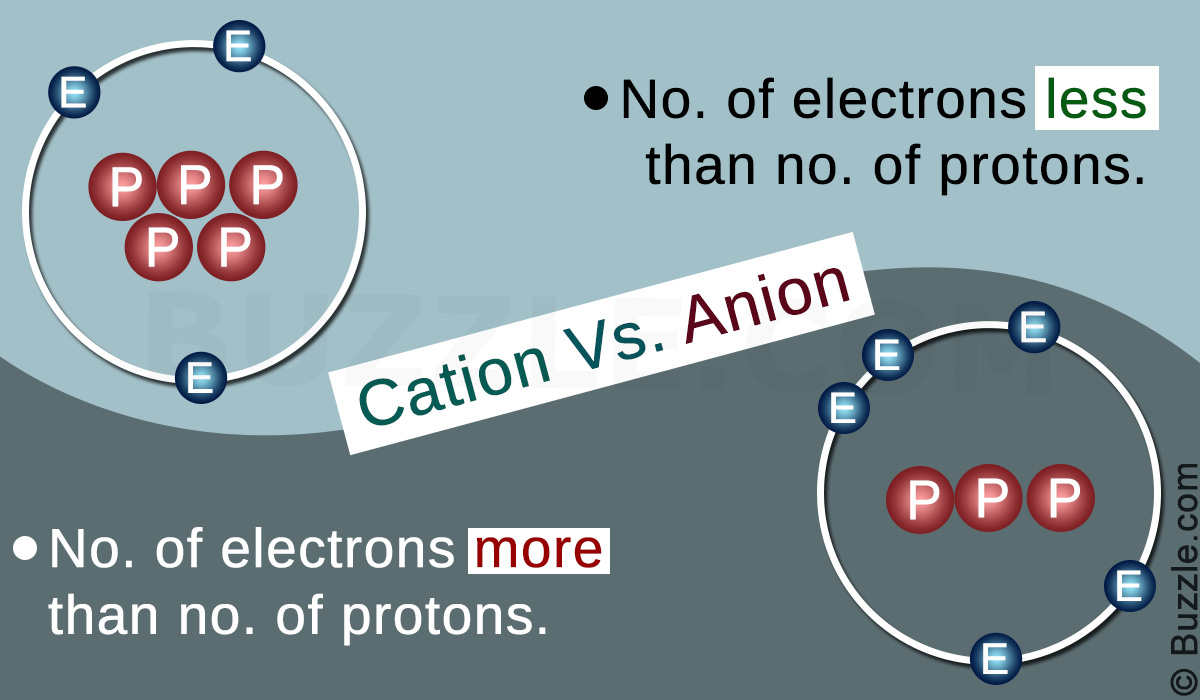
As mentioned above, a cation is formed when an atom gives away electrons. The atomic nucleus consists of protons which are positively charged. An atom, in its original form, has an equal number of electrons and protons. Electrons are negatively charged and has a charge similar to that of a proton. Therefore, when an atom gives away electrons, it develops a relative positive charge, as the number of protons in the nucleus exceeds the number of electrons in the shell. Hence, cations are positively charged.
Cations are usually formed by metal atoms in an attempt to gain the noble gas configuration. The word cation in Greek means ‘down,’ which can be related to the lowering of the number of electrons. During the electrolysis processes, cations are attracted to the cathode as the cathode produces negative charges. Cations can be elemental or complex in nature. A cation complex will contain several elements grouped together which shares a positive charge. This is common among the d block elements. Furthermore, a single element can have several cations depending on their state of oxidation. i.e. singular charge, doubly charged, triply charged, etc. Some common cations include; Na+, Ca2+, Fe3+ etc.
What is an Anion
As mentioned in the introduction, an anion is formed when an atom acquires electrons. Therefore, due to the addition of these electrons, the number of electrons within the elemental shells exceeds the number of protons within the nucleus. As the protons are positively charged, and the electrons are negatively charged, the anion develops a negative charge.
Anions are usually formed by non-metal elements in an attempt to gain the noble gas configuration. The word anion in Greek means ‘up,’ which could be related to the increase in the number of electrons within the element. During the process of electrolysis, anions are attracted to the anode, as the anode produces positive charges. Anions can be elemental or complex in nature. An anion complex will contain several elements grouped together which shares the negative charge. This is common among the d block elements. Also, a single element can have several anions depending on their state of oxidation. i.e. singular charge, doubly charged, triply charged, etc. Some common cations include; F–, O2-, NO3– etc.
Difference Between Cation and Anion
Definition
A cationis a positively charged ion resulted by the release of one or more electrons from its shells in the attempt to increase stability
An anionis a negatively charged ion resulted by the acceptance of one or more electrons to its shells in the attempt to increase stability.
Charge
Cationsare positively charged.

Anionsare negatively charged.
Form of Atom
Cations are usually formed by metal atoms.
Anionsare usually formed by non-metal atoms.
Is Se A Cation Or Anion
Electrolysis
Cationsare attracted to the cathode (the end that produces negative charges).
Anionsare attracted to the anode (the end that produces positive charges).
Cation Vs Anion
Formation of Compounds
Cationsform electrostatic interactions with anions to form ionic compounds.
Anionsform electrostatic interactions with cations to form ionic compounds.
Image Courtesy:
Examples Of Anions And Cations
“Ions” by Jkwchui – Own work. (CC BY-SA 3.0) via Wikimedia Commons